Abstract
Background: The clinical course of immune thrombocytopenia (ITP) can be categorized into three phases: newly diagnosed (<3 months post-diagnosis), persistent (≥3-≤12 months) or chronic (>12 months). Having previously been restricted to use in patients with chronic ITP, romiplostim (a thrombopoietin receptor agonist) was recently approved in the USA and Europe for use across all phases of ITP in adult patients refractory to 1st line treatments. Its efficacy and safety in adult patients with ITP diagnosed for ≤6 months have been reported in a Phase 2 study (Newland, BJH 2016); however, its effect in patients with newly diagnosed ITP is not well characterized. Here we report post hoc analyses from the same Phase 2 romiplostim study stratified by duration of ITP diagnosis (<3 months and ≥3-≤12 months).
Methods: Adult patients with primary ITP diagnosed within 6 months prior to study entry and an insufficient response to first-line therapies (corticosteroids, intravenous immunoglobulin, anti-D immunoglobulin or vinca alkaloids) were enrolled in a Phase 2, interventional, single-arm study (NCT01143038). Patients had to have a single platelet count ≤30 × 10 9/L at any time during the 4-week screening period. Splenectomized patients, those who received rituximab, and those who had previously received romiplostim or other platelet-producing agents were excluded. Patients were to receive 12 months of romiplostim therapy at a starting dose of 1 µg/kg, with dose adjusted to target platelet counts ≥50-<200 ×10 9/L. Concomitant and rescue treatments were permitted to maintain platelet count. Following the 12-month treatment period, romiplostim dose was tapered for patients maintaining platelet count ≥50 × 10 9/L. Tapering could also begin earlier during the 12-month treatment period according to platelet counts and dose adjustment rules.
Efficacy outcomes included the duration (expressed as a percentage of months) with platelet response (defined as median platelet count ≥50 × 10 9/L during a month without rescue medication use in the previous 8 weeks) during the 12-month treatment period; treatment-free remission (TFR; weekly platelet count ≥50 × 10 9/L for 24 consecutive weeks with no ITP treatments); time to platelet response; durable platelet response (platelet response for ≥6 of the last 8 weeks of treatment during the first 24 weeks); and sustained platelet response (platelet response for 9 weeks in any 12-week period). Safety was assessed throughout the study. Post hoc analyses were performed by subgroups of ITP duration (newly diagnosed ITP: <3 months; persistent ITP ≥3-≤12 months).
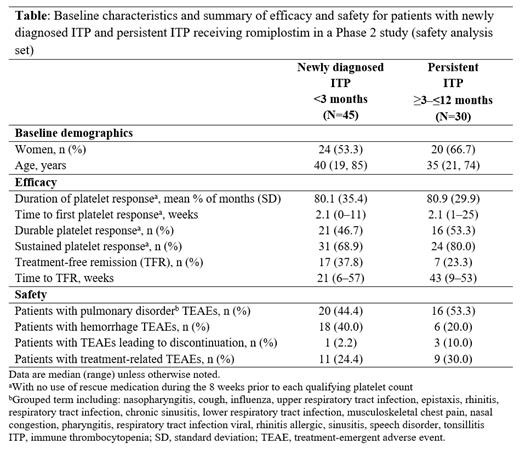
Results: Of 75 patients enrolled, 45 had newly diagnosed ITP, 30 had persistent ITP. Baseline demographic are shown in the Table. Mean (standard deviation; SD) duration of platelet response was 80.1% (35.4) of months for patients with newly diagnosed ITP and 80.9% (29.9) for patients with persistent ITP (Table). Median (range) weeks to first platelet response was 2.1 (0-11) in the newly diagnosed group and 2.1 (1-25) in the persistent ITP group. In the newly diagnosed ITP group 21 (46.7%) and 31 (68.9%) patients had durable and sustained platelet response, respectively; in the persistent ITP group, 16 (53.3%) and 24 (80.0%) patients had durable and sustained platelet response, respectively. TFR was achieved by 17 (37.8%) newly diagnosed patients and 7 (23.3%) persistent ITP patients. Median (range) weeks to TFR was 21 (6-57) in the newly diagnosed group and 43 (9-53) in the persistent ITP group. The safety profile of romiplostim was broadly similar across the two groups.
Conclusion: In this Phase 2 study of patients with primary ITP and disease duration of ≤6 months, romiplostim demonstrated efficacy irrespective of whether patients had newly diagnosed (<3 months duration) or persistent (≥3-≤12 months) disease. Efficacy outcomes were generally similar in both groups of patients, with rapid time to platelet response (~2 weeks) observed and around half of patients achieving a durable platelet response. Respective TFR rates were 37.8% and 23.3% in patients with newly diagnosed and persistent ITP. Safety was consistent across groups and no new safety signals were observed. This subgroup analysis was consistent with the previously reported overall findings of the study and supports the use of romiplostim for adult patients with primary ITP, including those with newly diagnosed and persistent disease.
Newland: GSK: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau; Grifols: Consultancy, Speakers Bureau; BMS: Research Funding; Argenx: Consultancy, Speakers Bureau; Angle: Consultancy; Amgen: Consultancy, Research Funding, Speakers Bureau; UCB Biosciences: Consultancy; Roche: Speakers Bureau; Octapharma: Research Funding. Viallard: LFB: Consultancy; Grifols: Consultancy; Novartis: Consultancy; Amgen: Consultancy. López Fernández: Amgen: Honoraria. Eisen: Amgen: Current Employment, Current equity holder in publicly-traded company. Saad: Amgen: Current Employment, Current equity holder in publicly-traded company. Hippenmeyer: Amgen: Current Employment, Current equity holder in publicly-traded company. Godeau: Novartis: Consultancy; Sobi: Consultancy; Amgen: Consultancy; Grifols: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal